Project Overview
The main goal of this
project is to present a design that is approachable by all. This
means it needs to be as simple as possible, built from
readily-available materials, using simple fabrication techniques, and
it must be low-cost. A bit lower on the priorities list is that it
too must be easy to operate and maintain, and must produce useful
output in reasonable quantities.
Biomass conversion techniques
involve large volumes and high temperatures. By its very nature then,
this project will involve metal fabrication, both sheet metal and
machining. The parts will not be complicated, though, so if you
cannot make them yourself, you should no have to go far to have them
made.
After reviewing the field, I have settled on a
thermochemical process called fast pyrolysis, which, its simplest
terms:
"Fast pyrolysis is a
high temperature process in which biomass is rapidly heated in the
absense of oxygen. As a result it decomposes to generate mostly
vapors and aerosols and some charcoal. After cooling and
condensation, a dark brown mobile liquid is formed,which has a
heating value about half that of conventional fuel oil."
('Fast
Pyrolysis of Biomass: A Handbook' A. Bridgewater ISBN:
1872691072)
OK. Fast. How fast? According to the sources I have read (ToDo:
Bibliography), the whole process needs to be done on the order of a
couple of seconds. That is, heating up the biomass to over 500C, and
cooling the resultant vapors and aerosols to condense them in 2
seconds. About the best way I can think to do this is by using a
fluidized-bed reactor (Figure 1)
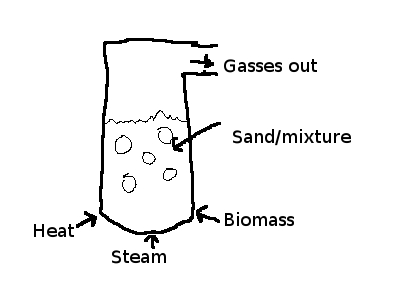
As
can be seen, a fluidized bed is a container filled with small
particles, (often sand, but can be any high-temperature small-scale
mixture) into which heat, biomass and sometimes steam are introduced.
Details of my thoughts for the reactor chamber can be found here.
The biomass is rapidly heated in such an environment, and turns to
gases and aerosols (entrained liquid in gas). In fast pyrolysis,
these product gases are rapidly cooled upon exiting the reactor.
These gases, upon cooling, condense to a mixture of various
compounds:
“The liquid contains
several hundred different chemicals in widely varying proportions,
ranging from formaldehyde and acetic acid to complex high molecular
weight phenols, anhydrosugars and other oligosaccharides.”
Further, large fractions of the product are problematic. The
product (by weight) is 10-15% Acetic acid, and 20-30% water.
The interesting thing (perhaps the perplexing thing) about this
product, is that:
“Pyrolysis
liquids cannot be completely vaporised once they have been recovered
from the vapour phase. If the liquid is heated to 100ºC or more
to try to remove water or distil off lighter fractions, it rapidly
reacts and eventually produces a solid residue of around 50wt% of the
original liquid and some distillate containing volatile organic
compounds and water.”
Hmmm, actually quite perplexing, for most methods of
separation/processing involve raising the temperature of the process
to facilitate any reaction. OK, I propose:
Do all post-creation processing of the the bio-gas while it is
still a gas
I propose, at the very least, a fractional condensation of
the product gases, meaning, cooling rapidly to the liquid point of
the first sought-after product. In the case of bio-oil, this would be
down to near the point of condensing acetic acid, around 118C,
or 140C for acetol, then down to just above 100C, to capture product
above water's boiling point, then finally 90C or so, to capture
water. The gases remaining can be re-introduced to the reactor.
Perhaps a picture will help (also see the Mechanical design page
here:
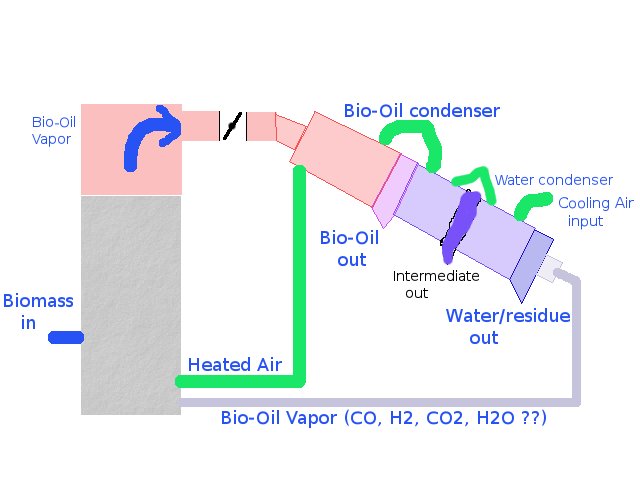
The aforementioned idea could be used to yield a highly dense
(little water present) biomass derivative, that could be titrated
with alcohols to produce a heating or transportation fuel.
This idea can be extended in both directions from the disclosed
temperature range for condensation.
If between the reactor and the first stage of condensation,
sensible heat, electromagnetic energy (light, UV, microwave), or
mechanical (in the form of ultrasound or other macro-molecular wave
phenomenon) is added to the product gas, many reactions are
possible, from steam reformation to large molecule cracking.
If the last stage is further refined, alcohols could be
stripped from the product stream, and the non-condensible gases
captured and put to use rather than recycle.
Revision : 30 Date: 2006/07/13 21:52:07

